A novel polymer material for industry
09 08 2025
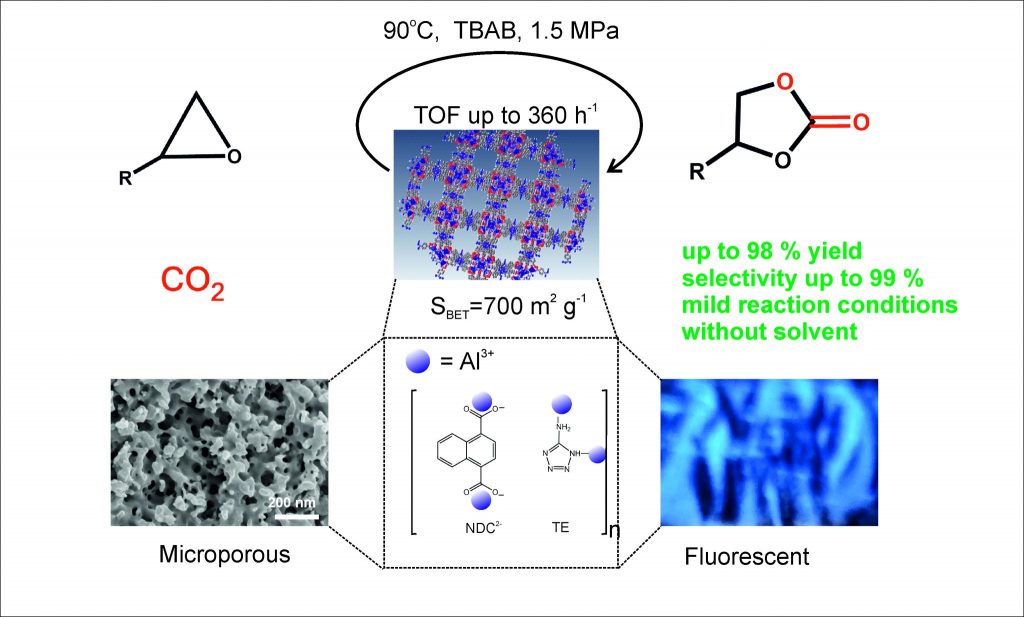
A novel method of coordination polymer fabrication for industrial applications has been developed at the University of Warsaw. This polymeric material adsorbs carbon dioxide and plays the role of a green catalyst in chemical reactions. During the reaction, carbon dioxide captured in polymer crystals is utilised for organic carbonates production.
A team of researchers (Gabriela Kopacka, M.Sc., Eng., and Kinga Wasiluk, M.Sc., Eng.) from the Faculty of Chemistry, University of Warsaw, led by Professor Elżbieta Megiel, has discovered a method for producing new polymeric materials capable of adsorbing carbon dioxide while also possessing catalytic properties. These materials belong to the group of highly porous coordination polymers (MOFs). The researchers know how to produce them using a simple method using commonly available and inexpensive substrates.
Eco-Friendly Material
“We have developed a new material that catalyses chemical reactions. Using various types of epoxides allows for the simple production of industrially valuable cyclic carbonates, without the use of toxic substances. This represents a breakthrough, as previously, industrial processes for producing organic carbonates have involved environmentally harmful methods. Our new material may enable a shift away from these practices and enable the future production of carbonates ecologically and safely,” says Professor Elżbieta Megiel.
The creators of the technology emphasise that the chemical reaction used in CO2 utilisation is characterised by 100% atomic efficiency, meaning that, as a waste-free method, it is exceptionally environmentally friendly. Thanks to the use of highly selective catalysts it allows for the production of products of very high purity. These include organic carbonates currently produced on an industrial scale for numerous applications (e.g., electrolytes in lithium-ion batteries, ingredients in cosmetics and pharmaceuticals, green solvents and substrates for the production of biodegradable polymers).
CO2 utilisation
The ecological aspect is crucial to the newly discovered technology. Materials with such high porosity (their specific surface area ranges from 400 to 700 m 2 per gram strongly adsorb carbon dioxide. This can be compared to the adsorption of water by a sponge. While adsorbing CO2, the crystals of these polymers do not lose their catalytic properties. Therefore, they can be used as catalysts in reactions in which CO2 becomes a substrate.
These nanostructures can be prepared in powder or compressed pellet form, can be used after CO2 adsorption in chemical reactors, where the trapped CO2 reacts with epoxides to form organic carbonates. This allows for its disposal. Using an epoxide derived from renewable materials, such as ethylene oxide, limonene oxide, or α-pinene oxide, which can be obtained from biomass, would be difficult to achieve a more ecological result. This way, we utilise waste CO2 based on the principles of sustainable development goals, explains Professor Elżbieta Megiel.
The type of organic carbonate produced by the developed catalysts depends on the type of epoxide used as the basis for the chemical reaction. Importantly, after the reaction is complete, the catalyst can be easily and fully recovered and reused, as it does not lose its catalytic properties after repeated use. The team of researchers proved the stability of the discovered nanomaterials up to 400°C. They can be stored in a normal atmosphere and a wide temperature range, and can also be reused multiple times.
Commercialization
The technology is patent-protected in Poland and abroad. Its commercialisation is being led by the Centre for Technology and Knowledge Transfer at the University of Warsaw. The creators plan to collaborate with industry representatives to conduct further testing and ultimately implement the solution.
“At this stage, we are committed to increasing the technological readiness of the invention. It demonstrates high effectiveness under controlled laboratory conditions, but to conduct full industrial-scale testing, we need an external partner. The team of creators is ready to collaborate with partners in the further stages of research and implementation,” says Professor Przemysław Dubel, Director of the Centre for Technology and Knowledge Transfer at the University of Warsaw.
CO2 adsorbing agents?
Because the discovered materials adsorb carbon dioxide from the air, they could potentially be used as new adsorbing agents for Carbon Capture and Utilisation (CCU) technologies. However, this material should be considered as one of many available options, as there are materials and substances on the market that are more effective for adsorbing CO2, such as polyethyleneimine and ethylenediamine. Nevertheless, in the case of amines, it is not possible to utilise CO2 directly to produce useful products, as is the case with the method developed at the University of Warsaw.
“I can imagine our technology ultimately being used to adsorb CO2, for example, from waste gases from industrial production or power plants, and then this material being directed to organic carbonates production lines. From a technical perspective, there are no contraindications or limitations. However, what’s important today is that potential partners take an interest in the new technology, test it on an industrial scale, and help implement it for widespread use,” adds Professor Elżbieta Megiel.
New polymeric materials are created by combining 1,4-naphthalenedicarboxylic acid, 4-aminotriazole, or 5-aminotetrazole with aluminium ion clusters. These reagents form highly porous crystalline structures. The raw materials required for the production of these polymers are widely available and inexpensive. The production of the new polymers is achieved through a simple, environmentally safe, and low-cost synthesis. In the epoxide carboxylation process, the new polymers serve as highly selective catalysts for the reaction, which results in the formation of useful cyclic organic carbonates from the epoxides and adsorbed carbon dioxide.

Check full article:
https://www.mdpi.com/1422-0067/25/2/1020