Article in JACS, ASAP Article (2025)
25 07 2025
Mechanism of pressure-induced hydrogen bond symmetrization in natrochalcite mineral revealed by experimental charge density study
Details of this mechanism are presented in the just published JACS paper:
Symmetrization of Strong Hydrogen Bond under High Pressure in Bihydroxide-Ion-Containing NaCu2(SO4)2·H3O2 Revealed by Experimental Charge Density, Single-Crystal Electron Diffraction, and Neutron Diffraction Studies, Piotr Rejnhardt*, Roman Gajda, Magdalena Woińska, Jan Parafiniuk, Gerald Giester, Ronald Miletich, Yan Wu, Tomasz Poręba, Mohamed Mezouar, Szymon Sutuła, Tomasz Góral, Przemysław Dera, and Krzysztof Woźniak*,
Journal of the American Chemical Society, ASAP Article (2025), https://doi.org/10.1021/jacs.5c08310
The context of work. Upon compression, all hydrous minerals, as well as other inorganic and molecular solids featuring hydrogen bonds, exhibit a peculiar common transformation involving the hydrogen atom. At pressures corresponding roughly to lower mantle depths, the distinction between the donor and acceptor in the hydrogen bond (HB) disappears, and the bond becomes symmetric and very strong. However, the nature of this phenomenon is not well understood due to a lack of systematic studies and the limitations of experimental methods sensitive to such subtle changes.
Specific problem addressed. Understanding the process of hydrogen bond (HB) symmetrization is essential, as the formation of strong symmetric HBs in hydrous minerals facilitates the transport of water to the deeper parts of Earth’s mantle¹ and significantly influences the superconductivity of hydrogen sulfide, which exhibits the highest critical temperature (Tc) measured to date.² In this study, we applied, for the first time, a charge density analysis based on high-pressure single-crystal diffraction data to gain insights into the mechanism of HB symmetrization in the structure of the natrochalcite mineral [NaCu₂(SO₄)₂·H₃O₂] under high pressure.
Analyzing electron density distributions refined against X-ray data under extreme conditions presents numerous challenges. The use of diamond anvil cells (DACs) in such experiments results in reduced resolution, low completeness, and limited quality of the X-ray data obtained. To address these limitations, very bright X-ray sources—such as modern synchrotron facilities using short-wavelength radiation—are employed to ensure high resolution and completeness of the data under high-pressure conditions.
Experiment, main results and conclusions. We performed a refinement of a quantitative multipole model of electron density against single-crystal synchrotron X-ray diffraction data collected at high pressure at the ID27 beamline. This approach allowed us to detect very subtle changes in electron density and provided deep insight into the detailed mechanism of hydrogen bond (HB) symmetrization.
The difference electron density maps revealed that at room temperature (RT) and ambient pressure, the hydrogen atom is disordered and split into two positions in the natrochalcite structure (phase I). However, at approximately 1.57 GPa, only a single positive electron density peak appears between the oxygen atoms (phase II), indicating that hydrogen atom ordering and HB symmetrization occur between 1.08 and 1.57 GPa.
At ambient pressure, the disordered hydrogen atoms are nearly collinear with the O(4) atom. As pressure increases (to 0.6 and 1.08 GPa), the electron density peaks shift apart, indicating a nonlinear configuration. Additionally, the disordered hydrogen atoms move off-center toward the oxygen atoms, forming a very short O–H covalent bond and a longer H···O hydrogen bond at 1.08 GPa (Figure 1).

Figure 1. Residual Fourier electron density maps showing the changes of electron density redistribution with pressure associated with disordered (phase I; atm – 1.08 GPa) and ordered (phase II; 1.57 – 3.65 GPa) hydrogen atom. The scales on the right side of the images represent values for the maximum and minimum peaks of residual density for maps in a particular row (eÅ–3).
The difference maps of the negative Laplacian values for hydrogen H(4B) illustrate regions where electron density increases with pressure (red iso-contours) and regions of charge depletion under compression (blue iso-contours), corresponding to pressure increments as shown at the top of the plots in Figure 2. We observed a significant concentration of electron density away from the O(4)···O(4) axis as pressure increases from ambient conditions to 0.6 and then to 1.08 GPa. This indicates that the shift of disordered hydrogen atoms to a nonlinear arrangement and off-center positions is driven by a notable redistribution of electron density—specifically, an outflow from the hydrogen bond center toward the oxygen atoms at 1.08 GPa.
However, as pressure increases from 1.08 to 1.57 GPa, a discontinuous electron density shift
occurs: electron density begins to accumulate in the previously depleted central region of the HB, and that area becomes electron-density-rich (Figure 2).
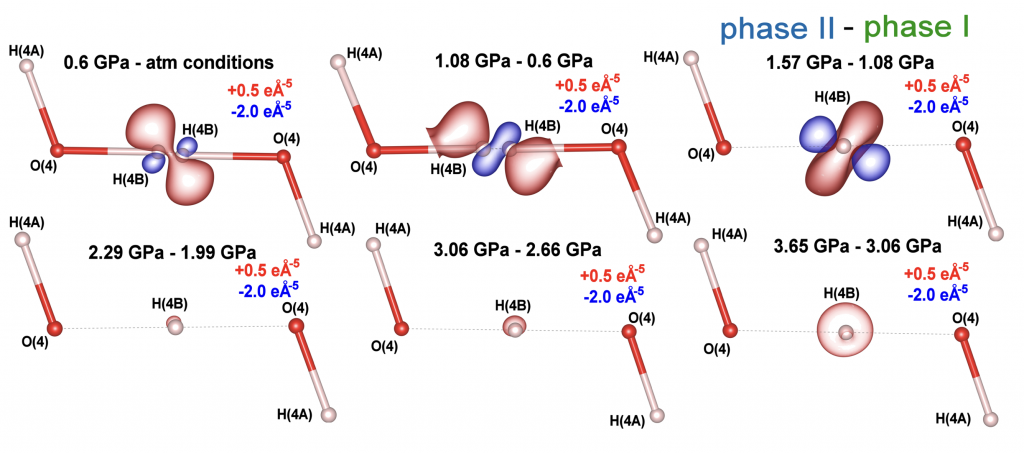
Figure 2. 3D maps of differences in negative Laplacian values for the hydrogen atom. These maps represent the difference in charge concentration and depletion between pressure points defined at the top of the particular figures. Red contours show regions of charge concentration, and iso-contours are at +0.5 eÅ–5. Blue contours showing regions of charge depletion, and iso-contours are at −2.0 eÅ–5. One can see complex reorganization of electron density in HB as a function of pressure.
We also attach (as separate 3 files: ) figures illustrating 3D distributions of negative Laplacian of electron density (contours at + 0.5 eÅ-5 and – 2.0 eÅ-5) versus differences in pressure (0.6GPa – atmospheric, 1.08GPa – 0.6GP and 1.57GPa – 1.08GPa, respectively). These figures illustrate concentration of electron density in the reddish area and depletion of electron density from the bluish area resulting from the increase of pressure. One can see switch of polarization of electron density between 1.08GPa and 1.57GPa. This is 3D version of the upper row of the above Fig. 2.
Figure 3(video). 3D maps of differences in negative Laplacian values for the hydrogen atom. These maps represent the difference in charge concentration and depletion between pressure points defined at the top of the particular figures. Red contours show regions of charge concentration, and iso-contours are at +0.5 eÅ–5. Blue contours showing regions of charge depletion, and iso-contours are at −2.0 eÅ–5. One can see complex reorganization of electron density in HB as a function of pressure.
One sentence summary. Experimental charge density redistribution revealed the mechanism of pressure-induced hydrogen bond symmetrization in natrochalcite mineral.
ESRF. In order to get extremely high quality of single crystal X-ray diffraction data we used microfocused X-ray beam, extra stabilised at the ID27 beamline during high pressure experiments. This is the only way to obtain at extreme pressure conditions high resolution and high completeness of X-ray data necessary to enable multipole refinement.
Conclusions. Our results demonstrate that hydrogen bond (HB) symmetrization is unambiguously a second-order phase transition. This process leads to a less compressible structure due to the formation of a symmetric HB with an unusually strong covalent character. Its formation significantly influences the physical properties of mineral structures under conditions typical of the Earth’s mantle, as well as in high-pressure hydrogen-rich superconducting systems.
HB symmetrization is preceded by continuous changes in the arrangement of disordered hydrogen atoms, including their counterintuitive off-center movement toward oxygen atoms under increasing pressure. We have captured the details of a complex reorganization of electron density within the strong HB as a function of pressure.
References
1. Zhu, S. C.; Hu, Q.; Mao, W. L.; Mao, H. K.; Sheng, H., Hydrogen-Bond Symmetrization Breakdown and Dehydrogenation Mechanism of FeO2H at High Pressure, J. Am. Chem. Soc. 2017, 139 (35), 12129– 12132, DOI: 10.1021/jacs.7b06528
2. Errea, I.; Calandra, M.; Pickard, C. J.; Nelson, J. R.; Needs, R. J.; Li, Y.; Liu, H.; Zhang, Y.; Ma, Y.; Mauri, F., Quantum Hydrogen-Bond Symmetrization in the Superconducting Hydrogen Sulfide System, Nature 2016 532:7597 2016, 532 (7597), 81– 84, DOI: 10.1038/nature17175
